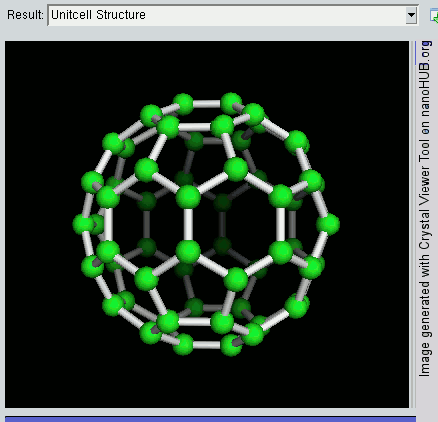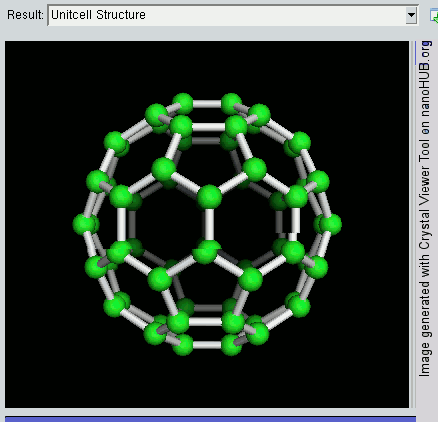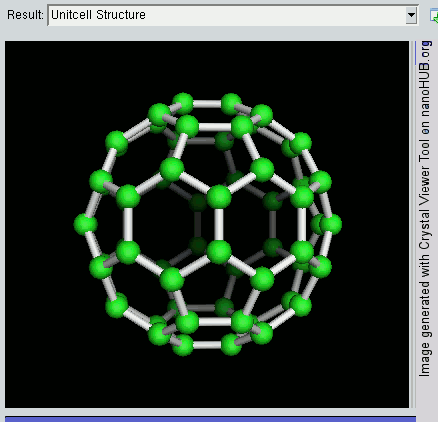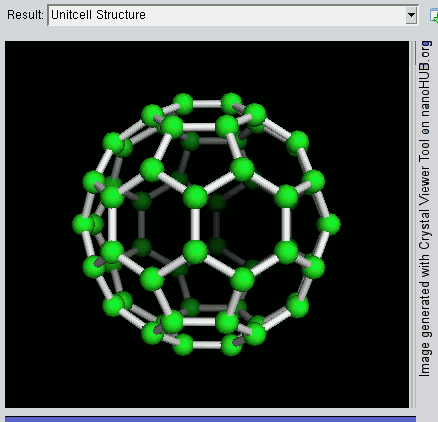
The buckyball is made up of 60 carbon atoms, linked together in such a way to form a molecule that looks like a soccer ball. C60 was first discovered by a group of scientists from the Rice University in 1985. They named it buckminsterfullerene, after Richard Buckminster Fuller, an American architect who was one of the first to design buildings that resembled the buckyball (geodesic domes). The scientists, Sir Harry Kroto, Bob Curl and Rick Smalley won the Nobel Prize in Chemistry in 1996 for their discovery.
Discovering the buckyball opened up an exciting field of study, called nanotechnology, which involves the building up of new materials, atom-by-atom. The buckyball is really strong, heat resistant, and is a superconductor -- its electrical resistance drops to zero when its temperature drops beyond a certain critical temperature. Some cool uses of the buckyball include a sponge to mop up free radicals in the brains of stroke victims, a supercomputer the size of a paperback novel, and in photocopier toners to improve the resolution of our photocopies.
Recently, NASA astronomers used the Spitzer Space Telescope and discovered these molecules in space, in a planetary nebula named Tc 1. Planetary nebulas are the remains of stars, like the sun, that shed their outer layers of gas and dust as they age. The buckyball is the largest molecule to be discovered in space so far. This was done by analysing the infrared light given off by the planetary nebula. This is a branch of technology and science known as spectroscopy.
I think the most amazing thing about this discovery is that the telescopes that astronomers have developed to date are able to analyse substances in space down to the molecular level, don't you think?
No comments:
Post a Comment