Thursday, July 29, 2010
The Science in Inception
I formally declare: Inception is now my new favourite movie. I love the whole concept of intruding dreams, bending reality and manipulating situations. Here is an article from New Scientist that discusses some of the neuroscientific concepts on which the movie is based.
If you haven't watched the movie, go watch it over the weekend if you have finished studying for your Lecture Test!
Sunday, July 25, 2010
Buckyballs in space!
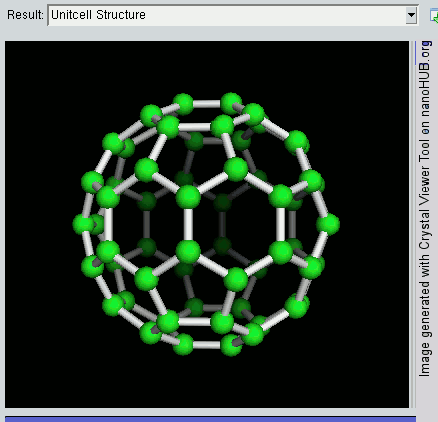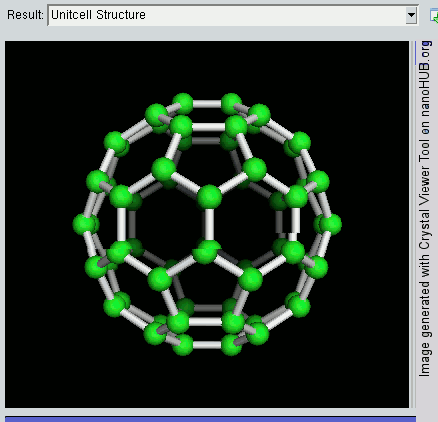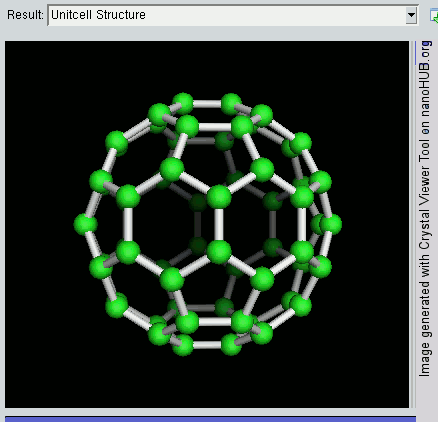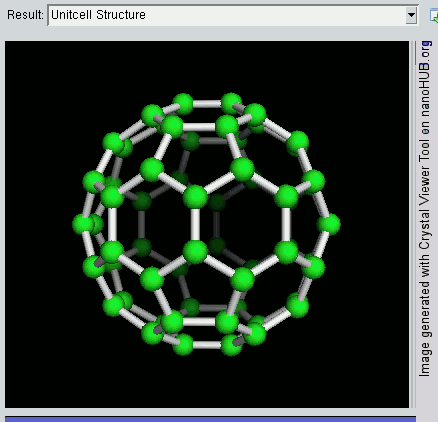
So far, we have become very good friends with two allotropes of carbon -- diamond and graphite. Allotropes are essentially elements in which the atoms have been arranged differently. As a recap, diamond and graphite are both made up of carbon atoms, but diamond adopts a tetrahedral lattice, but graphite has a layered structure. Let's meet another allotrope of carbon -- the buckyball.
The buckyball is made up of 60 carbon atoms, linked together in such a way to form a molecule that looks like a soccer ball. C60 was first discovered by a group of scientists from the Rice University in 1985. They named it buckminsterfullerene, after Richard Buckminster Fuller, an American architect who was one of the first to design buildings that resembled the buckyball (geodesic domes). The scientists, Sir Harry Kroto, Bob Curl and Rick Smalley won the Nobel Prize in Chemistry in 1996 for their discovery.
Discovering the buckyball opened up an exciting field of study, called nanotechnology, which involves the building up of new materials, atom-by-atom. The buckyball is really strong, heat resistant, and is a superconductor -- its electrical resistance drops to zero when its temperature drops beyond a certain critical temperature. Some cool uses of the buckyball include a sponge to mop up free radicals in the brains of stroke victims, a supercomputer the size of a paperback novel, and in photocopier toners to improve the resolution of our photocopies.
Recently, NASA astronomers used the Spitzer Space Telescope and discovered these molecules in space, in a planetary nebula named Tc 1. Planetary nebulas are the remains of stars, like the sun, that shed their outer layers of gas and dust as they age. The buckyball is the largest molecule to be discovered in space so far. This was done by analysing the infrared light given off by the planetary nebula. This is a branch of technology and science known as spectroscopy.
I think the most amazing thing about this discovery is that the telescopes that astronomers have developed to date are able to analyse substances in space down to the molecular level, don't you think?
Thursday, July 15, 2010
Molecular Cooking is Cooking: Molecular Gastronomy is a Scientific Activity
Have you heard of molecular gastronomy? Molecular gastronomy involves the application of scientific techniques into cooking. I tried some of this kind of food a few weeks back and I saw how the chef used liquid nitrogen to make meringue, and "fruit caviar" using compounds such as sodium alginate and calcium chloride.
This is a video of a lecture by a French Chemist, Herve This, given at Imperial College in London. Herve is a physical chemist (physical chemistry is essentially what we are learning now in JC1), whose main area of interest is molecular gastronomy. He is probably one of the leading chemists dealing with the art of culinary. It is a long video, but it is very entertaining to see him performing all sorts of procedures on egg white (or egg yellow, as he calls it) and explaining the Chemistry behind it. The french accent may be a little difficult to get used to, but I love how he constantly proclaims that "eet iz very eeazi".
Enjoy~
Tuesday, July 6, 2010
Cool stuff: The Ice-Calorimeter
From this week onwards, you will find yourself dealing with calorimeters during practicals, tutorials and lectures as we work through the topic of thermochemistry. You have handled the low-tech calorimeter made of styrofoam cups in the lab, and you have heard about a high-tech version called a bomb calorimeter. Have you wondered what the earliest calorimeters looked like and how they worked?
Here is a picture of the ice-calorimeter used by Antoine Lavoisier and Pierre-Simon Laplace to measure the enthalpy changes in 1785:
Here is a picture of the ice-calorimeter used by Antoine Lavoisier and Pierre-Simon Laplace to measure the enthalpy changes in 1785:
The chemists will place the reactants (the system) in the basket that you see in the middle of the calorimeter, and then pack ice (the surrounding) around the calorimeter. When the reactants react and produce heat, the ice around the calorimeter will melt. By measuring how much of the ice has melted, the chemists can then determine Q' and subsequently enthalpy change. However, as this deals with the melting of ice (change in state) instead of just an increase in temperature of water in the same state, the equations used to calculate Q' will be different. The Physics students should be able to figure this out.
Lavoisier and Laplace used this calorimeter to show that respiration (taking in oxygen to produce energy) is a form of slow combustion, or as Lavoisier put it "la respiration est donc une combustion". They achieved this by placing a guinea pig in the calorimeter and measuring the heat given out by the animal. The poor guinea pig.
The calorimeter in the photo is currently housed in the Science Museum in London. If you ever have a chance to go there, be sure to check it out. While at it, you can inform the other tourists of the fascinating Chemistry behind it.
Friday, July 2, 2010
Another new element!
In February this year, a new element, Copernicium was added to the periodic table. This element has a proton number of 112, and was at that time, the heaviest element to be officially added. Now, yet another element is on the verge of being included in this table -- element 114. So far, only two teams of scientists in Russia and America have been successful in producing this element, and in total 15 atoms (15 atoms, not 15 moles of atoms) of element 114 have been synthesised.
Element 114 is produced by combining calcium atoms and plutonium atoms. Each calcium atom has 20 protons, while each plutonium atom has 94 protons. When they fuse, they form a new atom with a total of 20 + 94 = 114 protons. Like Copernicium, atoms of element 114 are very radioactive and break down into smaller particles very quickly. On average, these atoms only existed for a few tenths of a second.
Now that the scientists are able to produce element 114, they have to discern the general chemical properties of this new element. To put it simply, they need to know whether they should classify element 114 as a metal or a noble gas. To do this, the scientists need to produce and capture these atoms, and test if they will adhere to a surface made of gold. If they do, element 114 is a metal. If they do not, element 114 is a noble gas as such atoms do not form any attractions with gold (Can you think of the reason why?).
Here is a video about this new element:
Subscribe to:
Posts (Atom)